
oomnia : Comprehensive software solution designed for clinical trials
oomnia: in summary
oomnia is a real-time SaaS platform that unifies all essential clinical trial tools-EDC, RTSM, CTMS, eTMF, eCOA, ePRO, and eConsent-into one cohesive system. With single sign-on, users save up to 75% of time spent navigating between systems. Its multi-tenant design allows stakeholders to manage multiple trials on a single instance and gain oversight of entire portfolios.
No coding is required due to intuitive drag-and-drop features. oomnia is fully interoperable, eliminating integration issues, and offers role-based access with a user-friendly interface customizable by language and notifications. Real-time reporting, seamless randomization, and complete audit logs enhance efficiency and data accuracy.
The platform complies with 21 CFR Part 11, EU Annex 11, and CDISC standards. ISO 27001 and ISO 9001 certified and FDA/Swissmedic inspected, oomnia enables up to 50% time and cost savings through streamlined, intelligent trial management.
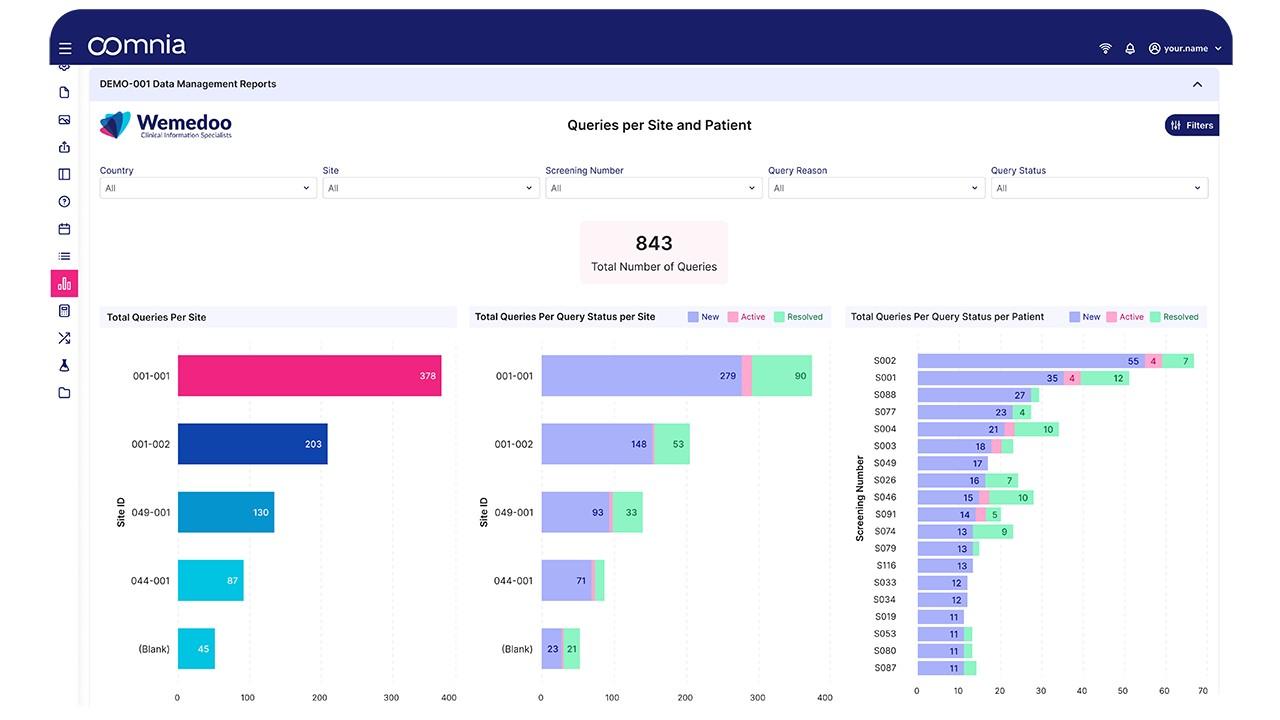 oomnia - Screenshot 1
oomnia - Screenshot 1 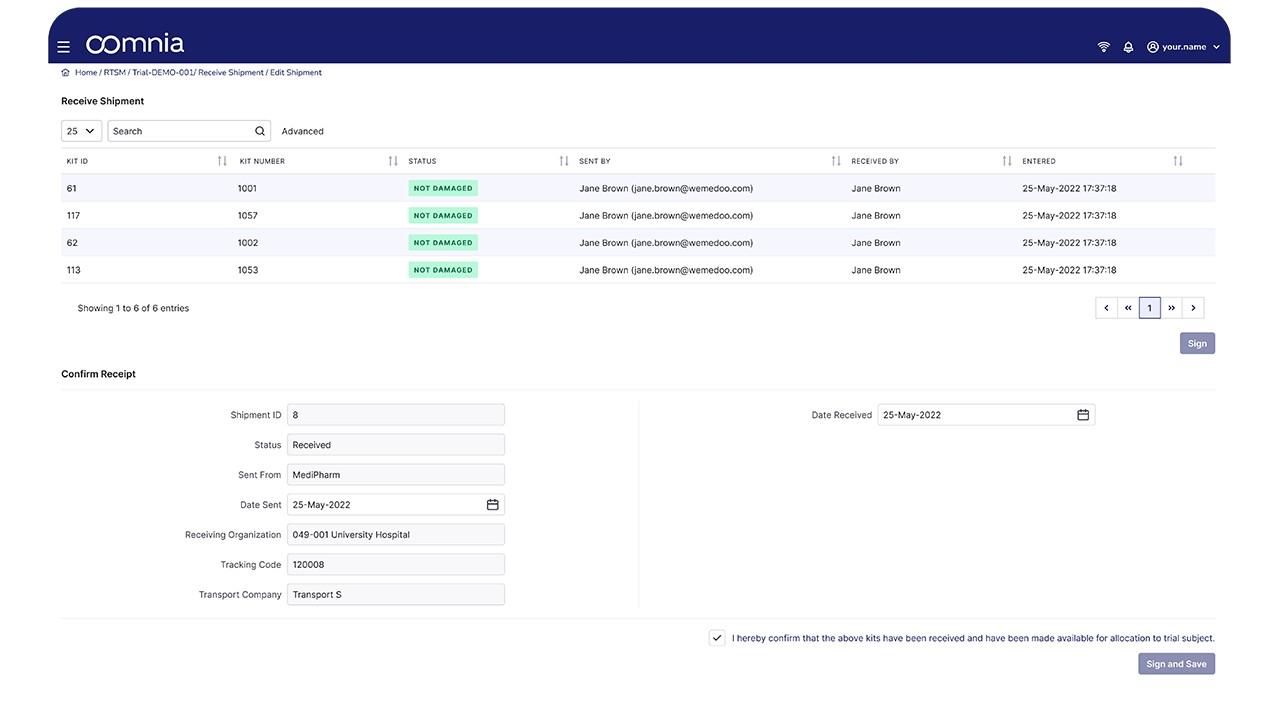 oomnia - Screenshot 2
oomnia - Screenshot 2 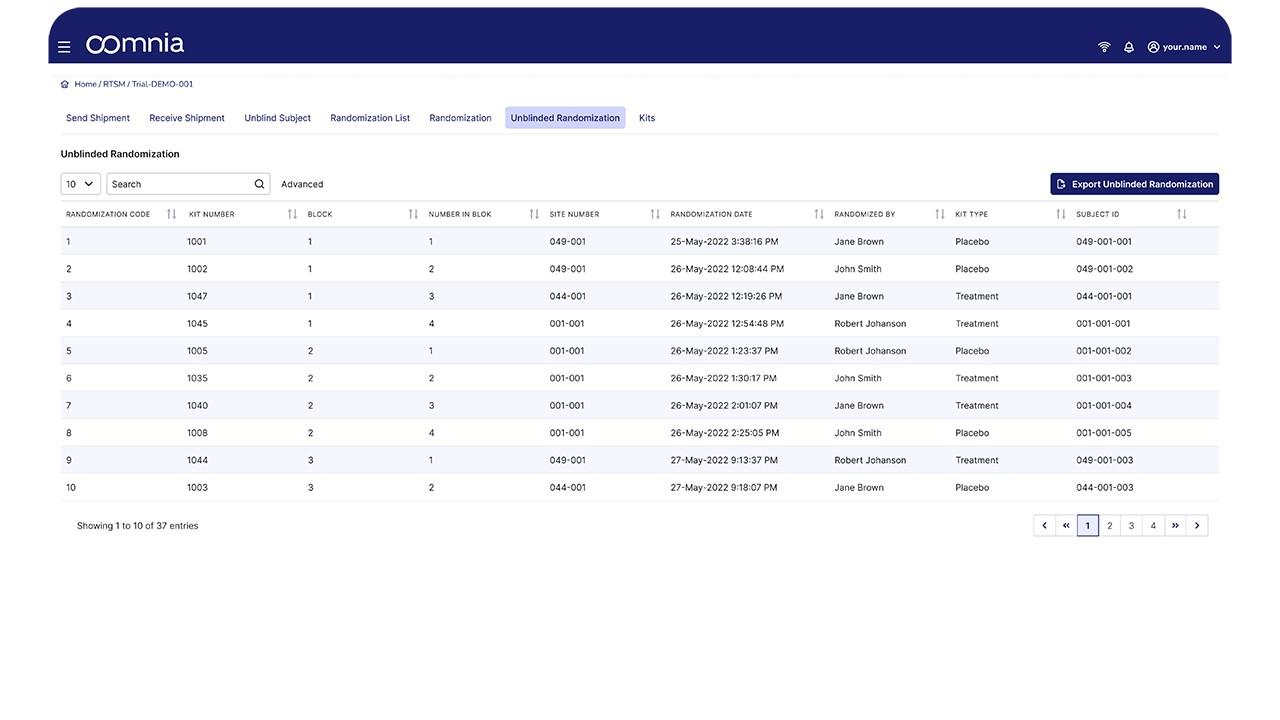 oomnia - Screenshot 3
oomnia - Screenshot 3 


oomnia: its rates
standard
Rate
On demand
Clients alternatives to oomnia

Streamline clinical trial management with software designed to optimize workflows, increase efficiency, and improve data accuracy.
See more details See less details
With advanced features like automated data capture, customizable dashboards, and real-time monitoring, this software simplifies trial management while providing greater visibility into study progress.
Read our analysis about i-OMS
Streamline clinical trial management with software designed to optimize workflows, track progress, and manage data.
See more details See less details
InClinicalPerform simplifies complex trial management tasks with intuitive features like automated reporting, customizable dashboards, and real-time data visualization. With seamless integration across teams and sites, the software ensures compliance and accelerates time-to-market.
Read our analysis about InclinicalPerform
Streamline clinical trial management with software that optimizes study planning, tracking, and reporting.
See more details See less details
Our clinical trial management software offers customizable templates, automated workflows, and real-time data tracking to ensure efficient study execution. With MATRIX CTMS, you can easily manage study budgets, track patient enrollment, and generate comprehensive reports for regulatory compliance.
Read our analysis about MATRIX CTMS Appvizer Community Reviews (0) The reviews left on Appvizer are verified by our team to ensure the authenticity of their submitters.
Write a review No reviews, be the first to submit yours.