
ClinPlus EDC : Streamline Clinical Trials with Advanced EDC Solutions
ClinPlus EDC: in summary
ClinPlus EDC is a powerful software designed for clinical research teams seeking efficient data management in clinical trials. Tailored for researchers and CROs, it boasts robust data collection, real-time access, and seamless integration, distinguishing it from other tools in the field.
What are the main features of ClinPlus EDC?
Enhanced Data Collection
The data collection capabilities of ClinPlus EDC are engineered to cater to the complexities of clinical trials, ensuring data accuracy and compliance with industry standards. With intuitive forms and customizable fields, this software transforms the data entry process, reducing labor-intensive tasks.
- Customizable electronic case report forms (eCRFs)
- Automated data validation checks
- Audit trails for robust documentation
Real-Time Access and Monitoring
ClinPlus EDC offers real-time access to clinical trial data, empowering research teams with actionable insights any time, anywhere. This feature enhances decision-making processes and facilitates on-the-go monitoring of study progress, ensuring timely interventions.
- Instant access to study metrics and reports
- Remote data review capabilities
- Real-time data updates from multiple sites
Seamless Integration with Existing Systems
Designed with integration in mind, ClinPlus EDC seamlessly connects with existing systems, ensuring a smooth data workflow and improving overall productivity. Its compatibility with other software ecosystems fosters an interoperable environment that strengthens research operations.
- Integration with laboratory information management systems (LIMS)
- Data exchange support with external databases
- API access for custom applications
 ClinPlus EDC - ClinPlus EDC-screenshot-0
ClinPlus EDC - ClinPlus EDC-screenshot-0 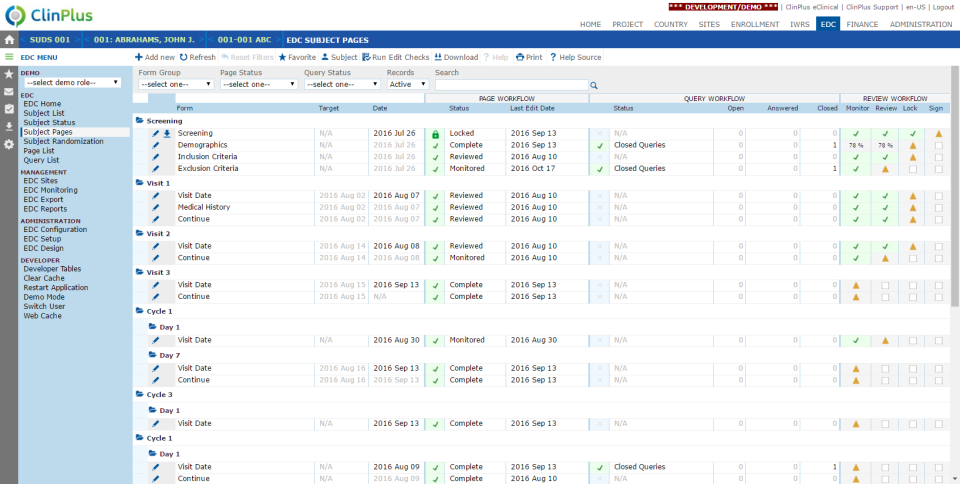 ClinPlus EDC - ClinPlus EDC-screenshot-1
ClinPlus EDC - ClinPlus EDC-screenshot-1 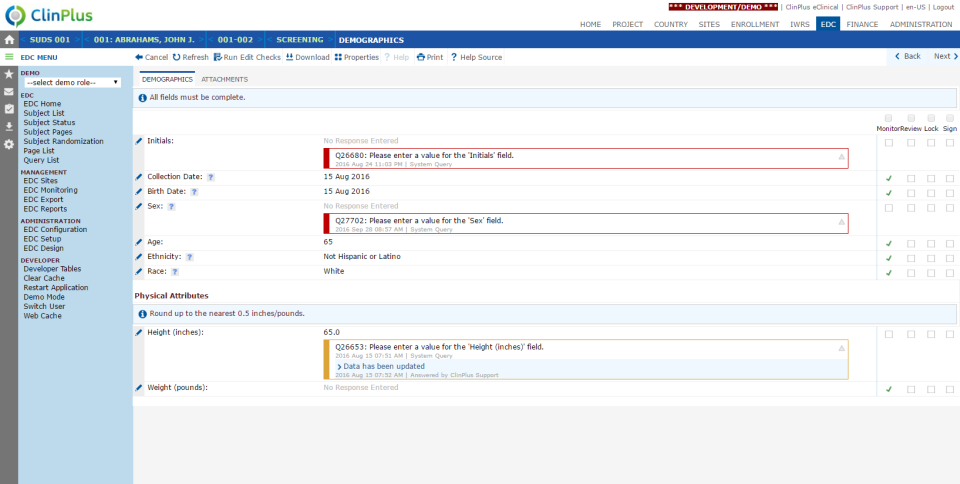 ClinPlus EDC - ClinPlus EDC-screenshot-2
ClinPlus EDC - ClinPlus EDC-screenshot-2 


ClinPlus EDC: its rates
standard
Rate
On demand
Clients alternatives to ClinPlus EDC

Streamline data collection with our Electronic Data Capture software. Simplify your research process and collect quality data efficiently.
See more details See less details
Our software provides a user-friendly interface for easy data entry and management. With customizable forms and real-time data validation, you can ensure accurate and complete data collection. Plus, our secure cloud storage ensures your data is safe and accessible from anywhere.
Read our analysis about i-CDMS
Streamline clinical trial data collection with advanced electronic data capture software.
See more details See less details
IBM Clinical Development offers efficient data management, real-time monitoring, and secure storage for clinical trials. Its user-friendly interface and customizable workflows simplify the data collection process, ensuring accuracy and compliance.
Read our analysis about IBM Clinical Development
Streamline your data capture process with cutting-edge software designed to automate data input and processing.
See more details See less details
Our Electronic Data Capture software is the perfect solution for businesses looking to automate their data entry and processing. With advanced features like optical character recognition and automated workflows, you can easily capture and process data from a variety of sources, including paper documents, emails, and web forms.
Read our analysis about Kofax Capture Appvizer Community Reviews (0) The reviews left on Appvizer are verified by our team to ensure the authenticity of their submitters.
Write a review No reviews, be the first to submit yours.